复宏汉霖H药 汉斯状 及汉曲优 多项研究数据发布
2024年9月18日,复宏汉霖(2696.HK)宣布,首个创新型单抗 H药 汉斯状 (斯鲁利单抗)、中美欧三地获批的曲妥珠单抗生物类似药汉曲优 的多项最新研究结果在2024欧洲肿瘤内科学会(ESMO)大会上以壁报形式展示。
H药 汉斯状 为复宏汉霖自主研发的重组人源化抗PD-1单抗注射液,也是全球首个获批一线治疗小细胞肺癌的抗PD-1单抗,已在中国、印尼、柬埔寨、泰国等地获批上市。以临床需求为导向,公司围绕H药进行了差异化、多维度的适应症布局,广泛覆盖肺癌、消化道肿瘤等高发大癌种。目前,H药已获批用于治疗微卫星高度不稳定(MSI-H)实体瘤、鳞状非小细胞肺癌(sqNSCLC)、广泛期小细胞肺癌(ES-SCLC)和食管鳞状细胞癌(ESCC)4项适应症,惠及患者约80,000人。其一线治疗ES-SCLC的欧盟上市许可申请(MAA)已获得欧洲药品管理局 (EMA) 受理,有望于2024年获批上市。此外,公司积极推进H药在更多差异化适应症中的创新联合疗法,如H药联合贝伐珠单抗及化疗一线治疗转移性结直肠癌(mCRC),联合化疗用于胃癌的新辅助/辅助治疗,联合化疗同步放疗治疗局限期小细胞肺癌(LS-SCLC)等。
汉曲优 (曲妥珠单抗,美国商品名:HERCESSI ,欧洲商品名:Zercepac )是复宏汉霖按照中国、欧盟和美国等生物类似药法规自主研发的曲妥珠单抗,也是公司首个通过FDA批准在美国开展商业化的产品,可用于治疗HER2阳性早期乳腺癌、转移性乳腺癌和转移性胃癌,涵盖原研已获批准的所有适应症。目前,汉曲优 已在全球48个国家和地区获批上市,累计惠及210,000+患者,为全球乳腺癌和胃癌患者带来可负担、高品质的治疗选择。
本次ESMO大会上发布的研究数据结果如下:
ASTRUM-005
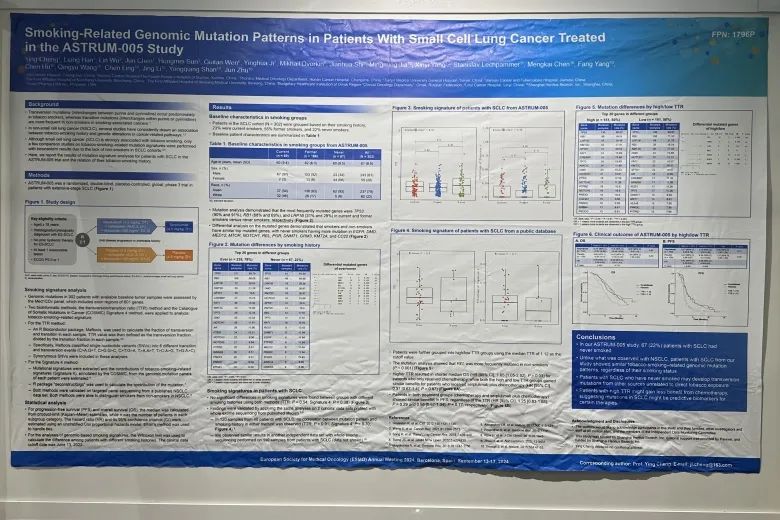
ASTRUM-005小细胞肺癌患者中吸烟相关突变的探索性研究
试验方法
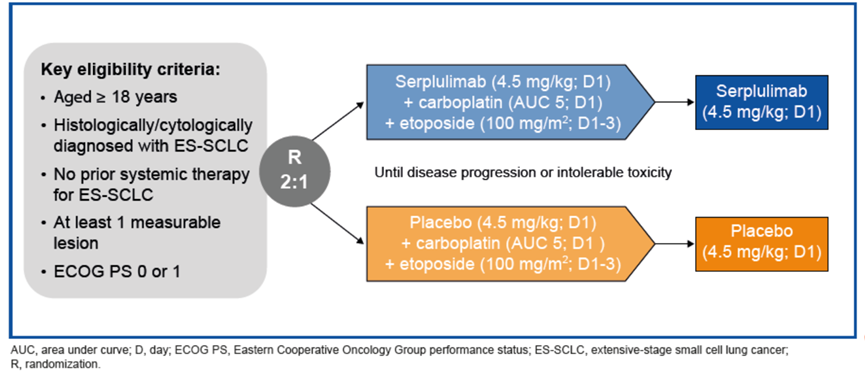
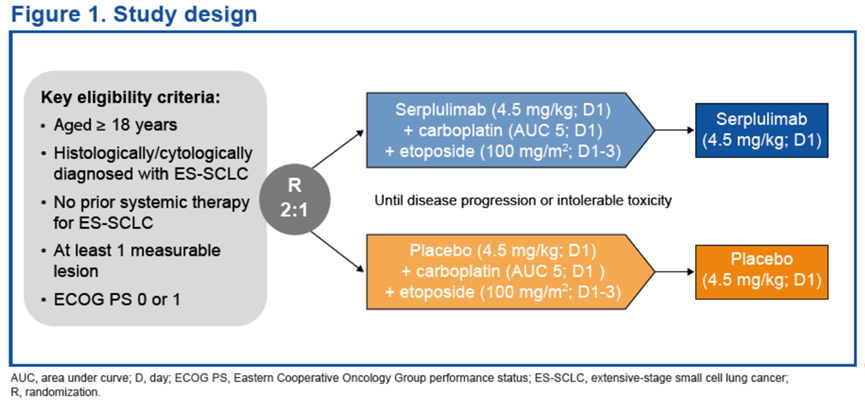
试验设计ASTRUM-005是一项针对广泛期小细胞肺癌(ES-SCLC)患者开展的随机、双盲、安慰剂对照的国际多中心III期临床试验。
吸烟特征分析
302名患者的基线肿瘤组织样本经 Med1CDxTM panel进行高通量测序分析基因突变,包括601个基因的外显子区。研究中利用两种生物信息学方法,即分别通过计算颠转(Transversion)和转换(Transition)比率(Ratio),以及吸烟相关突变占比(Cosmic数据库中Signature 4)来分析吸烟相关的基因突变特征,并采用Wilcoxon检验计算不同吸烟史患者之间的差异。在此前的非小细胞患者的分析研究中,这两种方法都可以观测到不同吸烟史患者之间的显著区别[1]。由于非吸烟的小细胞肺癌患者人数较少,之前未开展小细胞肺癌的相关研究。
TTR:使用Maftools计算每个样本中颠转(嘌呤置换嘧啶或嘧啶置换嘌呤的突变)及转换(嘌呤间或嘧啶间的置换突变)的数量,并计算这两种数量的比率(TTR值)。分析中包括了所有同义/非同义突变[2-3]
Signature 4:参考Cosmic数据库中对于不同突变特征的注释[4],使用R软件包deconstructSigs[5]提取各个样本的突变特征信息,并计算与吸烟相关突变特征(Signature 4)对于所有突变的占比。
统计分析
无进展生存期(PFS)和总生存期(OS)的中位数由乘积限(Kaplan-Meier)估计法计算得出,其中 n 是每个亚组类别中的患者人数。风险比(HR)及其95%置信区间(CI)采用未分层的Cox比例风险回归模型计算;并列关系使用Efron法处理。基因组的吸烟特征分析,使用 Wilcoxon 检验来计算不同吸烟史患者之间的差异。临床数据截止日期为2022年6月13日。
结果
吸烟组的基线特征
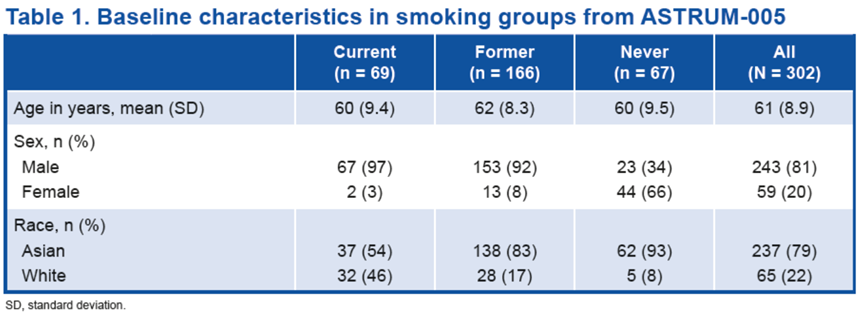
SCLC队列中的患者(N = 302)根据吸烟史进行分组;其 23%患者当前吸烟,55%患者曾经吸烟,22%患者从不吸烟。患者的基线特征汇总如下。
当前/既往吸烟组和不吸烟组患者的突变分析表明,最常发生突变的基因为 TP53 (90%和 91%)、RB1(68%和 69%)和 LRP1B(31%和 28%)。突变基因的差异分析表明,吸烟者与非吸烟者在常见突变基因方面相似,不吸烟者的常见突变基因包括EGFR、DMD、 MED12、MTOR、NOTCH1、REL、PGR、DNMT1、GRM3、KMT2A 和 CD22 等。
SCLC 患者的吸烟特征
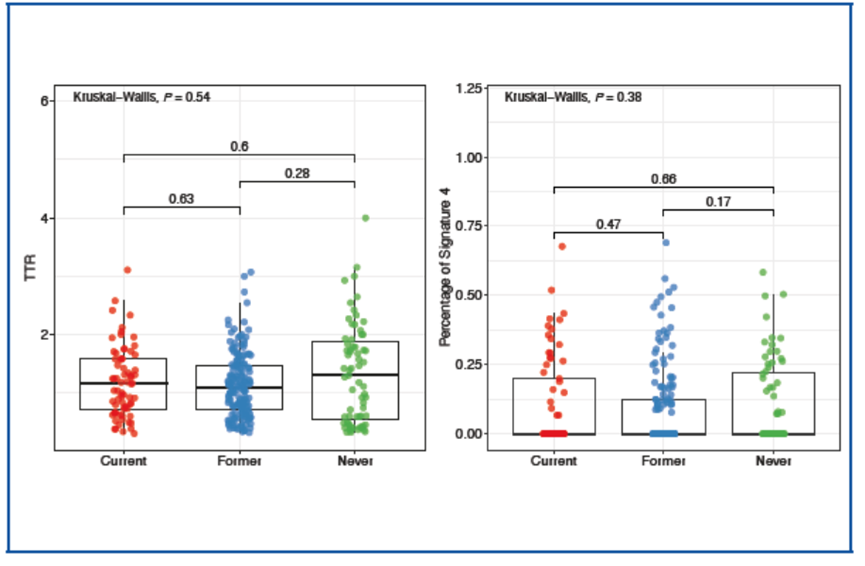
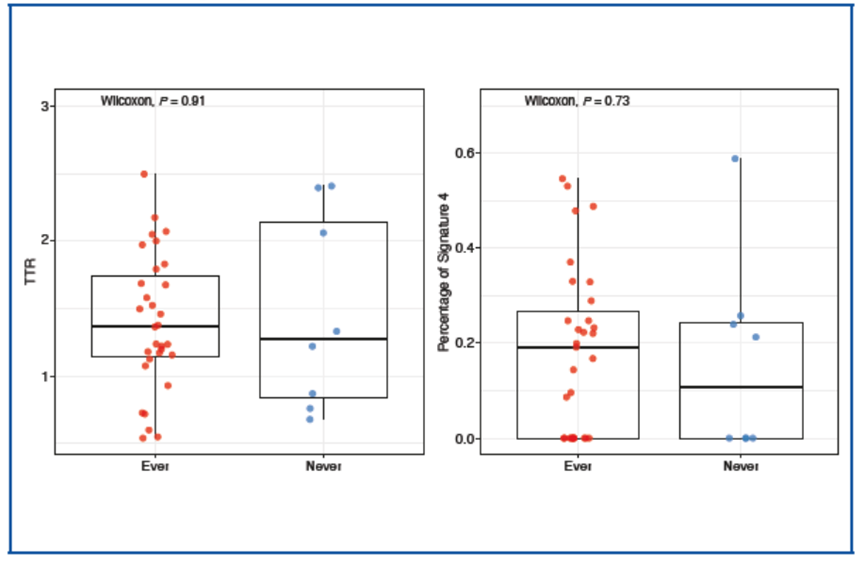
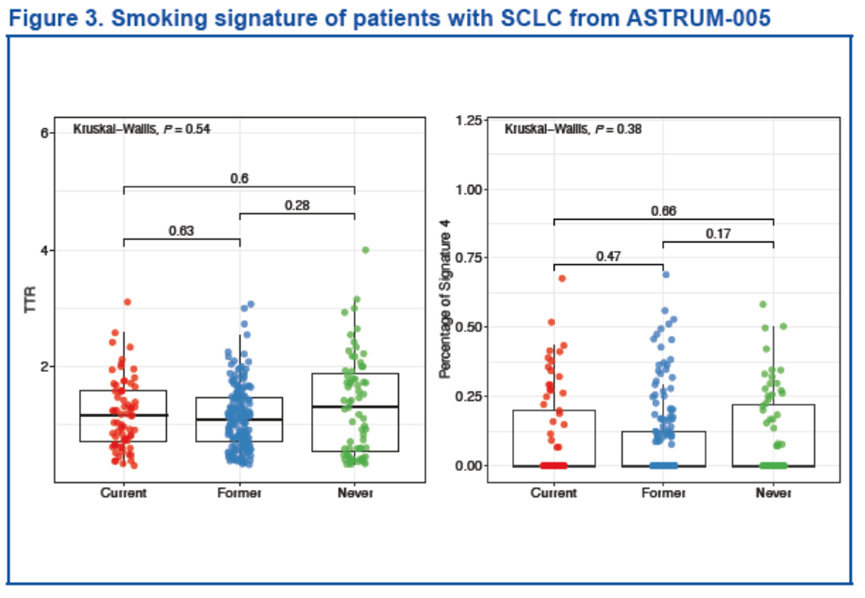
两种方法均未发现不同吸烟史组间的吸烟特征存在明显差异(TTR:P = 0.54;Sigature 4:P = 0.38)。
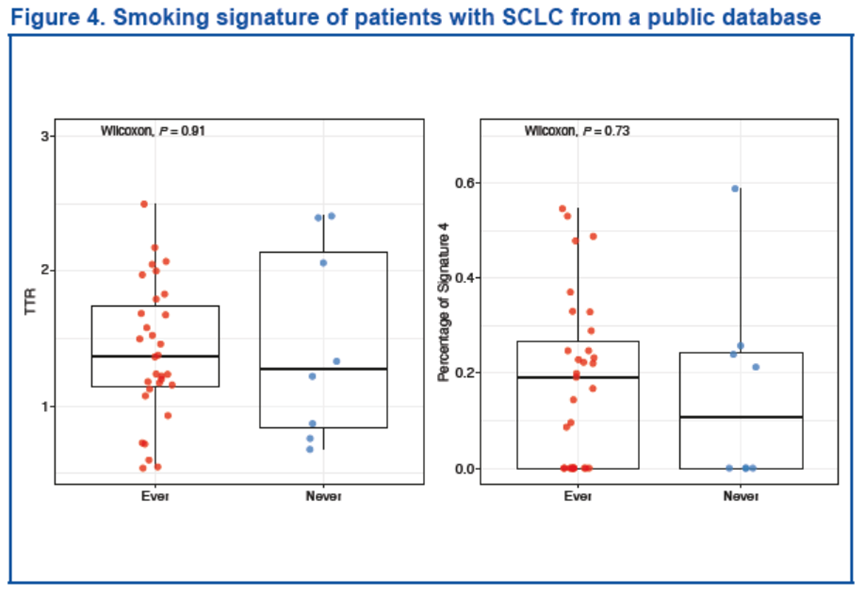
我们对既往研究中全外显子组测序分析的两个队列数据集进行相同的分析,验证了上述结果[1,6]。该研究中,来自 40 名 SCLC 患者的 120 份样本显示,无论采用哪种方法,都没有观察到突变模式与吸烟史之间的相关性。
在另一个独立的数据集中,我们对 110 例 SCLC 患者样本进行了全外显子组测序(数据未公布)[7],也观察到了相似的结果。根据 1.12 这一中位TTR值,我们将患者进一步分为高/低 TTR 组。突变分析表明,REL 在非吸烟者中突变频率更高(P < 0.001)。
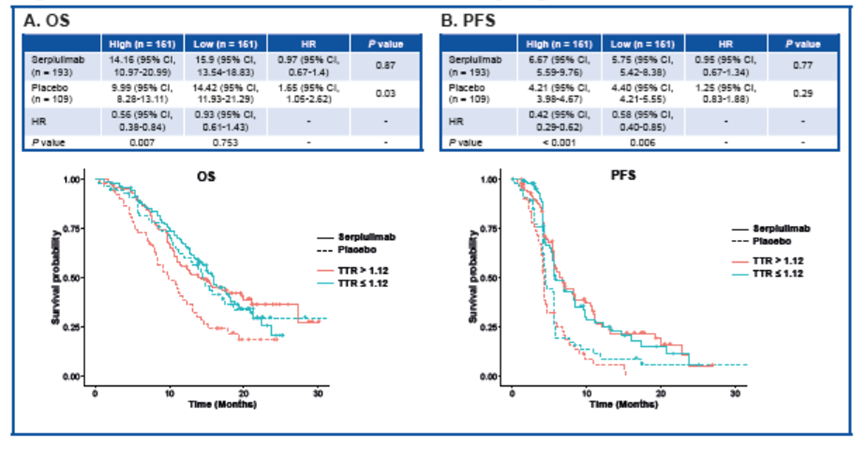
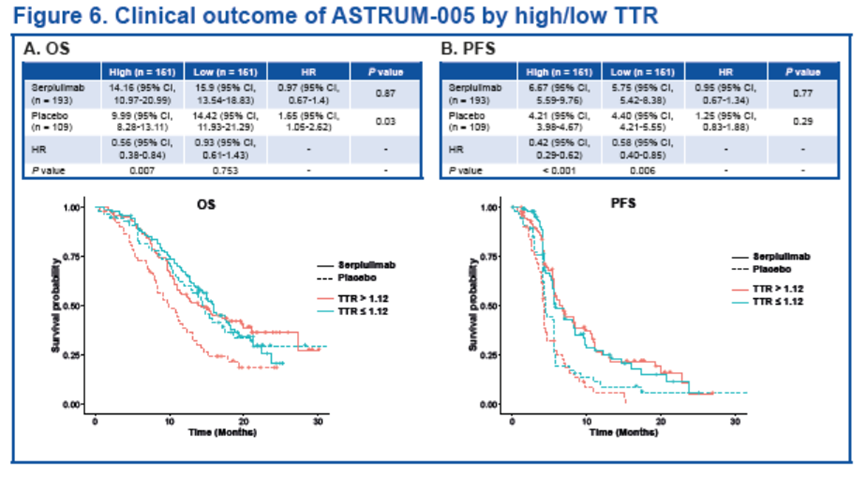
基于TTR值的分组,我们分析了不同组患者分的预后情况。对仅接受化疗的患者而言,TTR越高,中位OS则越短(HR [95% CI], 1.65 [1.05-2.62]; P = 0.03)。而对斯鲁利单抗联合化疗组的患者,高TTR和低TTR组间的临床获益相似(HR [95% CI],0.97 [0.67-1.4]; P = 0.87)。
无论TTR高低,两个治疗组(化疗组和斯鲁利单抗联合化疗组)的患者在PFS方面的获益皆相似。(HR [95% CI], 1.25 [0.83-1.88]; P = 0.29 和 0.95 [0.67-1.34]; P = 0.77)。
结论
本临床试验ASTRUM-005中共有67名(22%)无吸烟史的SCLC患者。我们的研究表明,SCLC患者无论吸烟与否,都存在类似的吸烟相关基因组突变特征,该发现与此前在NSCLC患者中的研究结果不同。这一研究结果提示,SCLC患者可能会发生与烟草直接接触无关的其他来源的基因颠换突变;且生存分析显示,高TTR的患者可能从化疗中获益较少,表明SCLC中的突变可以作为特定疗法的预测性生物标志物。
ASTRUM-004
斯鲁利单抗联合化疗一线治疗鳞状非小细胞肺癌的关键III期临床研究ASTRUM-004的探索性生物标志物分析
试验方法
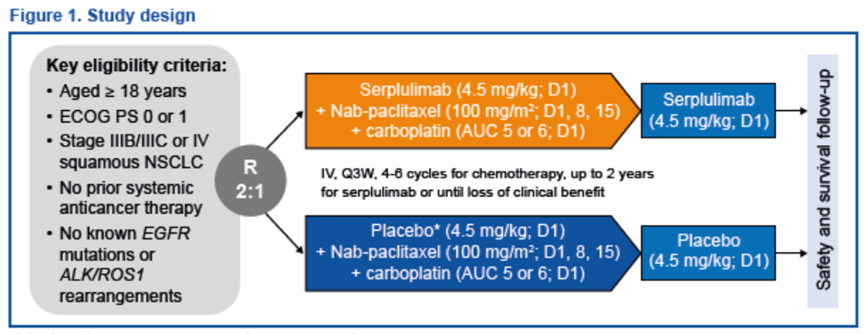
对符合要求的患者进行随机分组,按照2:1的比例接受斯鲁利单抗或安慰剂治疗,两者均与化疗联合使用。
生物标志物可评估人群(BEP)样本经 Med1CDxTM panel进行高通量测序分析基因突变。采用分层 Cochran-Mantel-Haenszel 法比较不同治疗组的客观缓解率(ORR),计算优势比(OR)及其 95% 置信区间(CI)。采用 Kaplan-Meier 法计算中位 PFS 和 OS。不同治疗组之间通过分层 Cox 比例风险回归模型估计风险比(HR)及其 95% CI。
结果
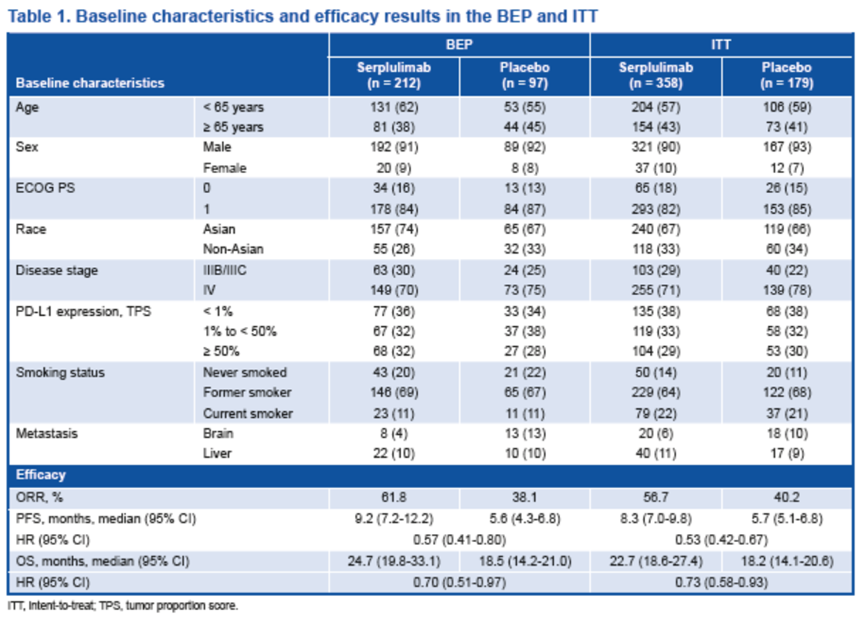
临床数据截止日期为 2023 年 1 月 31 日。共有 537 名符合条件的患者入组(斯鲁利单抗组358 人,安慰剂组179 人),309 名有可检测样本的患者被纳入BEP。
基线特征和基因突变情况
BEP的基线特征和疗效与ITT相当。
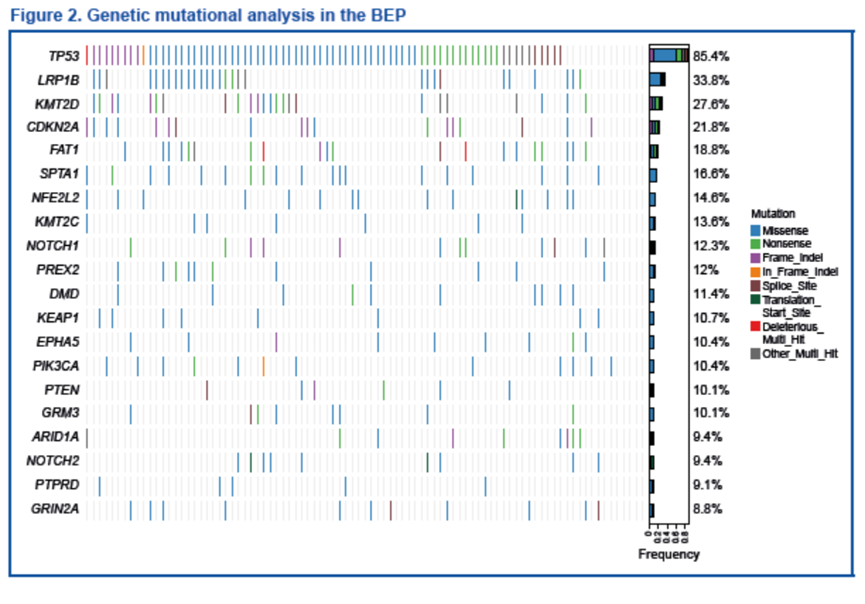
基因突变分析表明,最常见的突变基因为TP53(85%)、LRP1B(34%)和 KMT2D(28%),与既往报道的结果一致[8-10]。
突变情况的临床结果
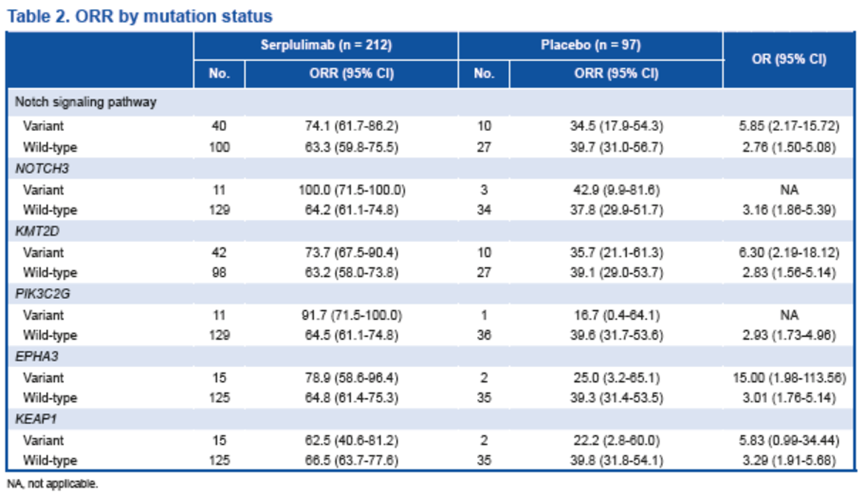
为确定斯鲁利单抗治疗的预测性生物标志物,我们分析了经斯鲁利单抗联合化疗治疗后,疾病缓解者(完全缓解或部分缓解的患者)和未缓解者(疾病稳定或进展患者)中不同突变频率的基因和信号通路,发现了Notch信号通路和其他一些基因的突变与斯鲁利单抗联合化疗更好的治疗效果相关。
无论基因突变情况如何,斯鲁利单抗治疗组患者的ORR始终高于安慰剂组的患者,存在上述基因突变患者的 ORR 有提高的趋势。
Notch信号通路中的基因突变,尤其是NOTCH3基因突变,与接受斯鲁利单抗治疗患者的中位无进展生存期(PFS)延长有关。与安慰剂组相比,斯鲁利单抗治疗组患者的PFS更长,这可能是因为它们在 sqNSCLC肿瘤微环境中发挥作用,这与之前的报道一致[11]。
结论
探索性生物标志物分析表明,无论基因突变情况如何,在化疗中联合斯鲁利单抗都能提高患者的临床获益。与没有基因突变的患者相比,Notch信号通路、KMT2D、PIK3C2G或EPHA3突变的患者可能会在斯鲁利单抗治疗中取得更高的临床获益。这些研究结果进一步支持斯鲁利单抗联合化疗作为一线sqNSCLC患者的标准治疗方案。
ASTRUM-LC01
ASTRUM-LC01:同步放化疗后斯鲁利单抗巩固治疗LS-SCLC
试验方法
这项多中心、II期临床试验(ASTRUM-LC01)纳入18~75岁、ECOG PS评分为0-1、接受4个周期标准化疗及同步大分割放疗(45Gy/3Gy/15F)和预防性颅脑照射(25Gy/2.5Gy/10F)后无进展的LS-SCLC患者,给予患者斯鲁利单抗巩固治疗直至疾病进展或不可耐受毒性,治疗最长至1年时间,旨在评估斯鲁利单抗巩固治疗LS-SCLC患者的客观缓解率(ORR)、 DpR (肿瘤缓解深度,定义为与诱导期相比肿瘤缩小的比例)、疾病控制率(DCR)、生存结局和安全性。
结果
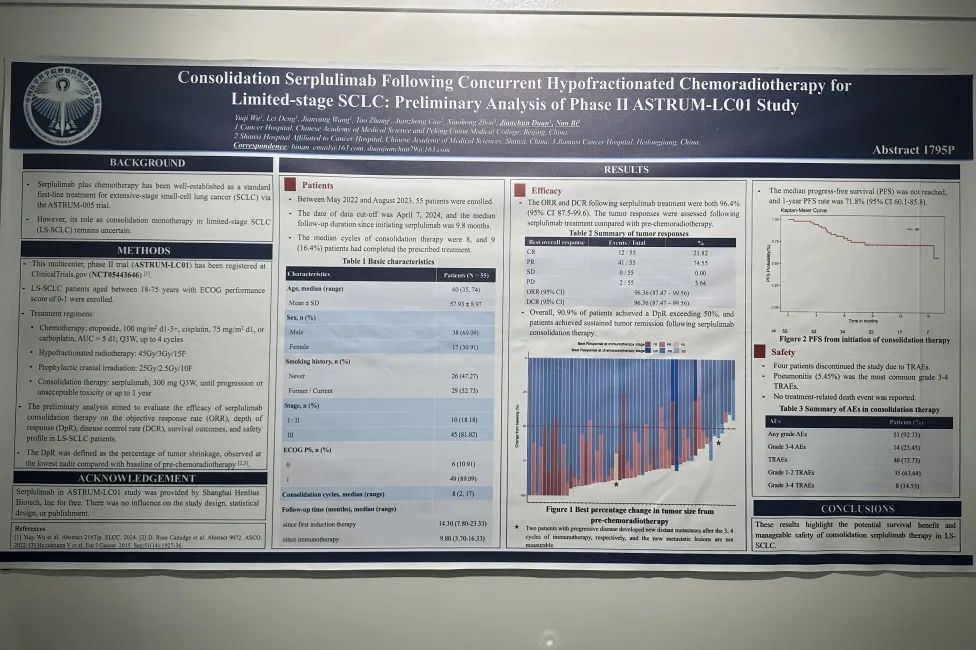
在2022年5月至2023年8月期间,研究入组55名患者。数据截止日期为2024年4月7日,自开始使用斯鲁利单抗起的中位随访时间为9.8个月。中位巩固治疗周期数为8个周期,9名(16.4%)患者完成了1年巩固治疗。
基线特征
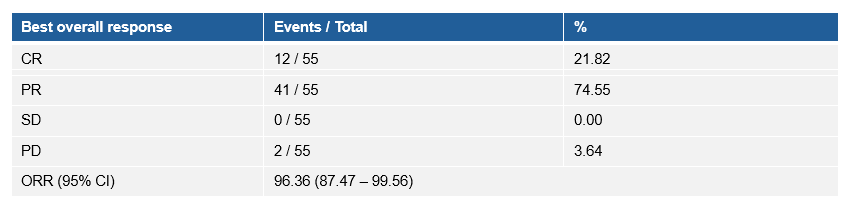
疗效数据显示,研究的ORR和DCR均为96.36%(与放化疗基线相比),90.9%的患者DpR超过50%,并且在接受斯鲁利单抗巩固治疗后,患者实现了持续肿瘤缓解。
肿瘤缓解
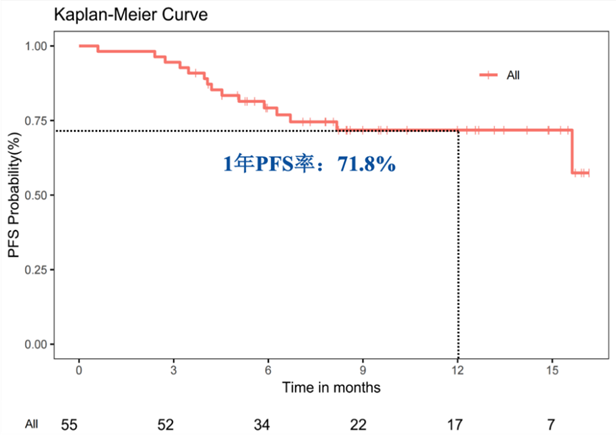
中位PFS和中位OS未达到,1年PFS率为71.8%(95% CI 60.1-85.8)。
巩固治疗开始后的PFS
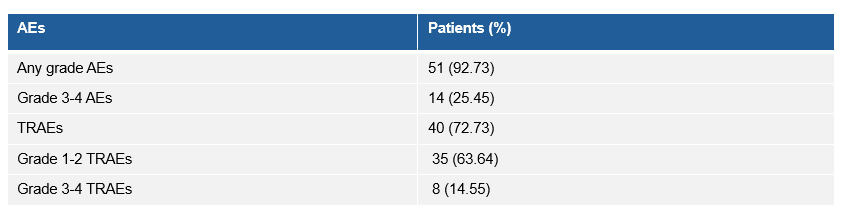
研究的整体安全性可控,3-4级的治疗相关不良事件(TRAE)发生率为14.55%,其中肺炎(5.45%)是最常见的3-4级TRAE,无治疗相关死亡事件发生。
安全性
结论
研究结果显示同步放化疗后斯鲁利单抗巩固治疗LS-SCLC具有显著的疗效获益和可控的安全性。
一项回顾性真实世界研究
免疫联合化疗及胸部放疗治疗ES-SCLC的疗效和安全性:一项回顾性真实世界研究
研究设计
这项在山东省肿瘤医院开展的单中心研究,回顾性分析2022年1月1日至2023年12月31日接受治疗的828 例 ES-SCLC 患者。根据治疗情况,将患者分为放化疗组(队列A) 接受4-6个周期化疗后进行胸部放疗、免疫化疗组(队列B) 接受4-6周期免疫联合化疗后免疫维持、免疫化疗+放疗组 (队列C)接受4-6周期免疫联合化疗后免疫+cTRT维持治疗。采用倾向评分匹配(PSM) 调整基线差异。主要研究终点为rwPFS和OS,次要终点为安全性。
结果
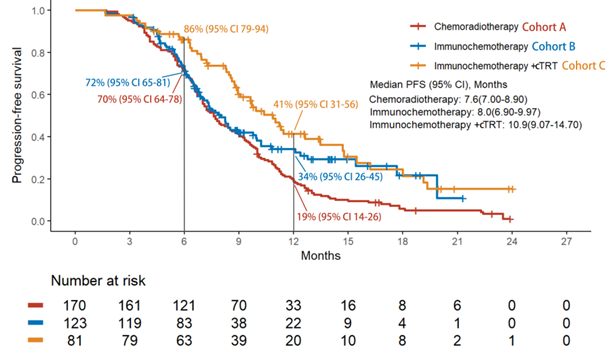
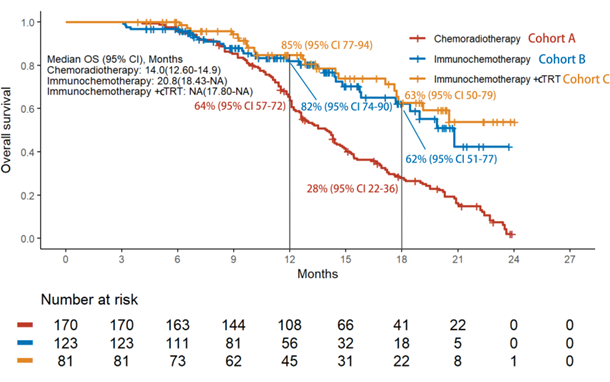
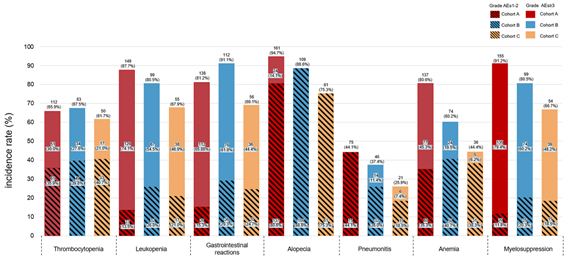
纳入分析374例患者,接受斯鲁利单抗治疗的患者110例(53.9%)。疗效结果显示,免疫化疗+放疗组的rwPFS和OS较放化疗组显著改善,免疫化疗+放疗组的中位rwPFS优于免疫化疗组和放化疗组(10.9个月vs 8.0个月 vs 7.6个月),免疫化疗+放疗组的1年rwPFS率高于免疫化疗组和放化疗组(41% vs 34% vs 19%),免疫化疗+放疗组和免疫化疗组及放化疗组的中位OS分别为NA vs 20.8个月vs 14个月,免疫化疗+放疗组的18个月OS率高于免疫化疗组和放化疗组(63% vs 62% vs 28%)。倾向性评分匹配分析结果显示,相比于放化疗组,免疫化疗后放疗组仍然能够显著提升患者的mPFS和mOS。安全性数据表明,≥3级不良事件的发生率在各组间相当,以骨髓抑制最为常见,≥3级肺炎的发生率在免疫化疗组和免疫化疗+放疗组明显较高,与既往报道一致。
PSM校正前三个队列的rwPFS结果
PSM校正前三个队列的OS结果
三个队列不良反应发生情况
结论
免疫联合化疗序贯胸部放疗治疗ES-SCLC的rwPFS和OS均有改善,总体安全性可控。未来可进一步开展前瞻性研究以确认在ES-SCLC治疗策略中联合cTRT的最佳方法。
一项开放标签的单臂II期临床研究
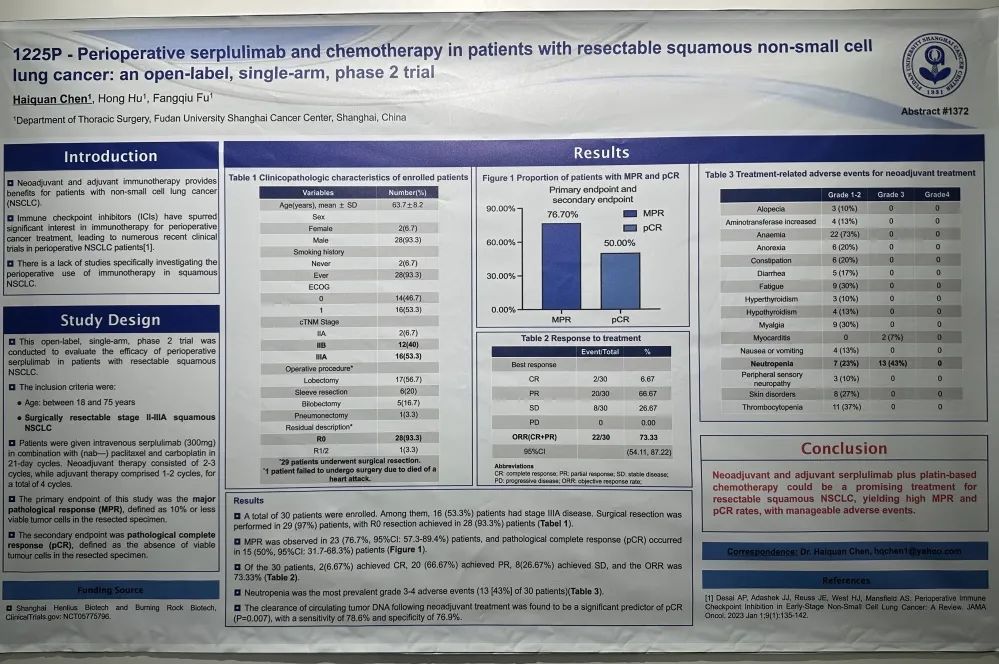
斯鲁利单抗联合化疗围手术期治疗可切除的鳞状非小细胞肺癌患者:一项开放标签、单臂、II期临床研究
研究设计
这是一项开放标签、单臂、II期研究,旨在评估斯鲁利单抗围术期治疗可切除鳞状NSCLC患者的疗效。纳入18~75岁可手术切除的II-IIIA期鳞状NSCLC,患者给予斯鲁利单抗(300mg)联合白蛋白紫杉醇和卡铂新辅助治疗2-3个周期,辅助治疗为1-2个周期,共4个周期。主要终点是主要病理缓解(MPR),次要终点是病理完全缓解(pCR)。
结果
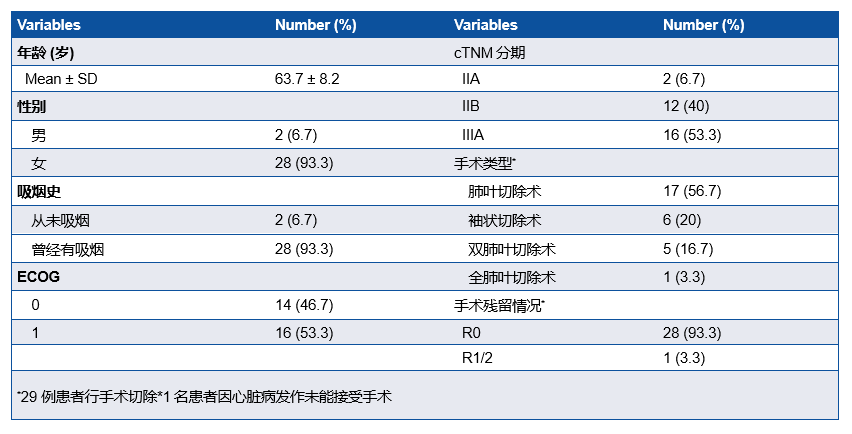
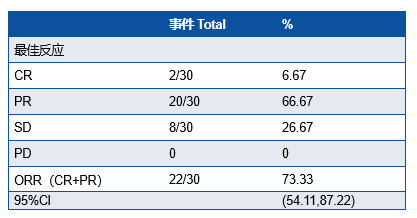
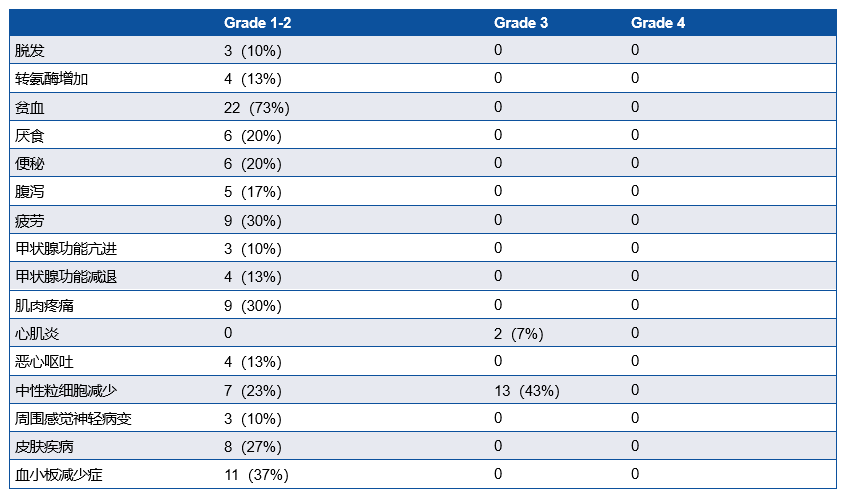
研究共纳入30例患者,IIIA期患者有16例(53.3%),29例患者行手术切除,28例(93.3%)患者达到R0切除(表1)。MPR率为76.7%, pCR率为50%。ORR为73.33%,其中2例(6.67%)患者达到CR(表2)。中性粒细胞减少症(43%)是最常见的3-4级不良事件 (表3)。新辅助治疗后循环肿瘤DNA清除率是pCR的重要预测因子(P=0.007),敏感性为78.6%,特异性为76.9%。
基线特征
MPR及pCR结果
治疗效果
新辅助治疗的治疗相关不良事件
结论
斯鲁利单抗联合含铂化疗新辅助和辅助治疗是可切除鳞状NSCLC的一种有希望的治疗方法,具有高MPR率和pCR率,安全性可控。
HER2 阳性 II-III 期乳腺癌新辅助治疗的 II 期研究
吡咯替尼联合白蛋白紫杉醇及曲妥珠单抗HLX02用于HER2 阳性 II-III 期乳腺癌新辅助治疗的 II 期研究
试验方法
先前未经治疗的非转移性HER2阳性BC患者被分配接受6个新辅助周期,每日一次口服吡咯替尼(400 mg),加HLX02 (8 mg/kg负荷剂量,随后是6 mg/kg)和nabp (260 mg/m2)。主要终点为pCR (ypT0/isN0)。在基线(P0)、新辅助治疗后(P1)和手术后(P2)使用metafer - se - fish全自动CTC图像扫描与分析系统捕获和计数CTC,并在BZ-X800显微镜采集图像后使用Imaris 10.1进行分析。
结果
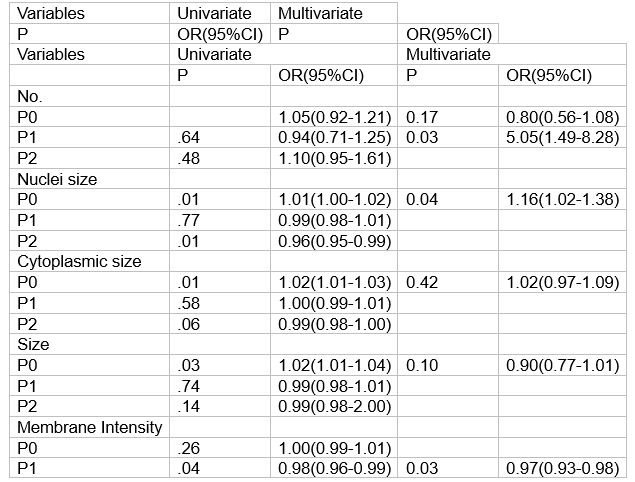
从2020年12月至2023年8月,共有214例患者入组并完成新辅助治疗和手术。pCR率为62.96% (95% CI, 55.65% ~ 69.86%,p=.001)。最常见的3-4级不良事件是腹泻(34.7%)和中性粒细胞减少(6.5%)。在95例接受ctc分析的患者中,63例(66.3%)实现了pCR。与P0组相比,P1组ctc计数明显减少(3.06±3.38 vs.0.84±1.53 FU/5ml, p< 0.001)。与非pcr组相比, pCR患者在P0时细胞核大小较大(95% CI, 1.02-1.38;P =.04),较少细胞计数(95% CI, 1.49-8.28;P =.03)和较低的膜强度(95% CI,0.93-0.98;P =.03)。
HER2阳性II-III期乳腺癌的单因素和多因素pCR分析
结论
在HER2阳性,II-III期BC的女性中,吡咯替尼+ HLX02和nabp新辅助方案有效地促进了肿瘤的pCR,并呈现出可接受的耐受性。在早期BC的新辅助治疗期间对CTC进行个性化监测可能有助于实时评估治疗反应并有助于预测pCR。
Latest Results of HANSIZHUANG and HANQUYOU Released at 2024 ESMO Shanghai, China, September 18, 2024 – Shanghai Henlius Biotech, Inc. (2696. HK) announced that the results on its first innovative product HANSIZHUANG (serplulimab) as well as its trastuzumab biosimilar approved in China, Europe and U.S., HANQUYOU were released as poster presentations at the 2024 European Society of Medical Oncology (ESMO) Congress.
HANSIZHUANG (serplulimab) is a recombinant humanised anti-PD-1 monoclonal antibody(mAb)injection independently developed by Henlius, and the world's first anti-PD-1 mAb approved for the first-line treatment of SCLC. The product has been approved in China, Indonesia, Cambodia and Thailand. Underpinned by the patient-centric strategy, Henlius has carried out a differentiated and multi-dimensional layout in the field of gastrointestinal cancer and lung cancer, covering a wide variety of indications. Up to date, HANSIZHUANG has been approved by the National Medical Products Administration (NMPA) for the treatment of MSI-H solid tumours, sqNSCLC, ES-SCLC, and esophageal squamous cell carcinoma (ESCC), benefiting about 80,000 patients. Meanwhile, HANSIZHUANG’s Marketing Authorisation Application (MAA) for ES-SCLC has been validated by the European Medicines Agency (EMA), which is expected to be approved in 2024. Moreover, a wide variety of clinical trials on immuno-oncology combination therapies in differentiated indications has been initiated by the company to further explore the efficacy of the product, such as HANSIZHUANG plus bevacizumab and chemotherapy as first-line treatment for patients with metastatic colorectal cancer (mCRC) , HANSIZHUANG plus chemotherapy as neoadjuvant/adjuvant therapy for gastric cancer (GC), and HANSIZHUANG plus chemotherapy and concurrent radiotherapy in patients with limited-stage small cell lung cancer (LS-SCLC), etc.
HANQUYOU is the trastuzumab biosimilar independently developed by Henlius in accordance with the National Medical Products Administration (NMPA), the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA) and other international biosimilar guidelines. It is Henlius’ first FDA-approved product. HANQUYOU is indicated for the treatment of HER2-positive early breast cancer, metastatic breast cancer and metastatic gastric cancer, which corresponds to all the approved indications of the trastuzumab originator. Up to date, HANQUYOU has been approved in 48 countries and regions, benefiting 210,000+ patients, bringing affordable and high-quality treatment options to breast cancer and gastric cancer patients worldwide.
ASTRUM-005
Smoking-related genomic mutation patterns in patients with small cell lung cancer treated in ASTRUM-005 study Methods ASTRUM-005 was a randomized, double-blind, placebo-controlled, global, phase 3 trial in patients with extensive-stage SCLC.
Smoking signature analysis Genomic mutations in 302 patients with available baseline tumor samples were assessed by the Med1CDx panel, which included exon regions of 601 genes. Two bioinformatic methods, the transversion/transition ratio (TTR) method and the Catalogue of Somatic Mutations in Cancer (COSMIC) Signature 4 method, were applied to analyze tobacco-smoking–related signature.
For the TTR method: An R Bioconductor package, Maftools, was used to calculate the fraction of transversion and transition in each sample. TTR value was then defined as the transversion fraction divided by the transition fraction in each sample[2-3]. Specifically, Maftools classified single nucleotide variants (SNVs) into 6 different transition and transversion events (C>A:G>T, C>G:G>C, C>T:G>A, T>A:A>T, T>C:A>G, T>G:A>C). Synonymous SNVs were included in these analyses.
For the Signature 4 method: Mutational signatures were extracted and the contributions of tobacco-smoking–related signatures (Signature 4), annotated by the COSMIC, from the genomic mutation panels of each patient were estimated[4].6 R package “deconstructSigs” was used to calculate the contribution of the mutation[5]. Both methods were validated on targeted panel sequencing from a published NSCLC data set. Both methods were able to distinguish smokers from non-smokers in NSCLC[6].
Statistical analysis For progression-free survival (PFS) and overall survival (OS), the median was calculated from product-limit (Kaplan–Meier) estimates, while n was the number of patients in each subgroup category. The hazard ratio (HR) and its 95% confidence interval (CI) were estimated using an unstratified Cox proportional hazards model; Efron’s method was used to handle ties. For the analyses of genomic-based smoking signatures, the Wilcoxon test was used to calculate the difference among patients with different smoking histories. The clinical data cutoff date was June 13, 2022.
Baseline characteristics in smoking groups Patients in the SCLC cohort (N = 302) were grouped based on their smoking history; 23% were current smokers, 55% former smokers, and 22% never smokers. Baseline patient characteristics are summarized in Table 1.
Mutation analysis demonstrated that the most frequently mutated genes were TP53 (90% and 91%), RB1 (68% and 69%), and LRP1B (31% and 28%) in current and former smokers versus never smokers, respectively. Differential analysis on the mutated genes demonstrated that smokers and non-smokers have similar top mutated genes, with never smokers having more mutation in EGFR, DMD, MED12, MTOR, NOTCH1, REL, PGR, DNMT1, GRM3, KMT2A, and CD22.
Smoking signatures in patients with SCLC No significant differences in smoking signatures were found between groups with different smoking histories using both methods (TTR: P = 0.54; Signature 4: P = 0.38) .
Findings were validated by applying the same analyses on 2 cohorts’ data sets profiled with whole exome sequencing from published studies[1,6]. In 120 samples from 40 patients with SCLC, no correlation between mutation pattern and smoking history in either method was observed (TTR: P = 0.91; Signature 4: P = 0.70)[7].
We observed similar results in another independent data set with whole exome sequencing performed on 110 samples from patients with SCLC (data not shown).10 Patients were further grouped into high/low TTR groups using the median TTR of 1.12 as the cutoff value. The mutation analysis showed that REL was more frequently mutated in non-smokers (P < 0.001) .
Higher TTR resulted in shorter median OS (HR [95% CI], 1.65 [1.05-2.62]; P = 0.03) for patients who only received chemotherapy, while both the high and low TTR groups gained similar benefits for patients who received serplulimab plus chemotherapy (HR [95% CI], 0.97 [0.67-1.4]; P = 0.87) .
Patients in both treatment groups (chemotherapy and serplulimab plus chemotherapy) showed similar benefits in PFS, regardless of the TTR (HR [95% CI], 1.25 [0.83-1.88]; P = 0.29 and 0.95 [0.67-1.34]; P = 0.77, respectively) .
Conclusion In our ASTRUM-005 study, 67 (22%) patients with SCLC had never smoked. Unlike what was observed with NSCLC, patients with SCLC from our study showed similar tobacco-smoking–related genomic mutation patterns, regardless of their smoking status. Patients with SCLC who have never smoked may develop transversion mutations from other sources unrelated to direct tobacco exposure. Patients with high TTR might gain less benefit from chemotherapy, suggesting mutations in SCLC might be predictive biomarkers for certain therapies.
ASTRUM-004
Exploratory biomarker analysis of phase 3 ASTRUM-004 study: serplulimab plus chemotherapy as first-line treatment for advanced squamous non-small-cell lung cancer Methods Eligible patients were planned to be randomized (2:1) to receive serplulimab or placebo, both in combination with chemotherapy.
Genetic mutations were assessed by the Med1CDx panel in the biomarker evaluable population (BEP). The objective response rate (ORR) was compared between treatment arms and the odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated using a stratified Cochran–Mantel–Haenszel method. Median PFS and OS were estimated using the Kaplan–Meier method. Comparisons between treatment arms were performed, and hazard ratios (HR) and their 95% CIs were estimated by a stratified Cox proportional hazards model.
The clinical data cutoff date was January 31, 2023. A total of 537 eligible patients were enrolled (358 patients in the serplulimab group and 179 patients in the placebo group); 309 patients with detectable samples were included in the BEP.
Baseline characteristics and gene mutation status Baseline characteristics and efficacy in the BEP were comparable with those in the ITT.
Mutation analysis demonstrated that the most frequently mutated genes were TP53 (85%), LRP1B (34%), and KMT2D (28%), consistent with published data[8-10].
Clinical outcomes by mutation status In order to identify predictive biomarkers for serplulimab treatment, we analyzed genes and signaling pathways with different mutation frequencies in responders (patients showing complete response or partial response) and non-responders (patients with stable disease or progressive disease) to serplulimab plus chemotherapy treatment. Mutations in the Notch signaling pathway and several other genes were identified. The ORR was consistently higher in patients treated with serplulimab versus placebo, regardless of gene mutation status. A trend of greater ORR was observed in patients treated with serplulimab who had these mutations.
Mutations in the Notch signaling pathway, especially for NOTCH3, were associated with longer median PFS in patients receiving serplulimab versus placebo, possibly due to their roles in the tumor microenvironment in sqNSCLC, which was consistent with previous findings.[11] Mutations in KMT2D, which is involved in the modulation of chromatin structure, and PIK3C2G and EPHA3, which regulate the tumor microenvironment, were associated with better clinical outcomes in the serplulimab arm compared with the placebo arm.
Conclusion The exploratory biomarker analysis suggests improved clinical benefit with the addition of serplulimab to chemo regardless of genetic mutation status. Furthermore, comparing to those without mutations, patients with mutations in Notch signalling pathway, KMT2D, PIK3C2G, or EPHA3 may derive more clinical benefit when serplulimab was added.
ASTRUM-LC01
Consolidation Serplulimab Following Concurrent Hypofractionated Chemoradiotherapy for Limited-stage SCLC: Preliminary Analysis of Phase II ASTRUM-LC01 Study Methods LS-SCLC patients aged between 18-75 years with ECOG performance score of 0-1 were enrolled.
Treatment regimens:
-Chemotherapy: etoposide, 100 mg/m2 d1-3+, cisplatin, 75 mg/m2 d1, or carboplatin, AUC = 5 d1; Q3W, up to 4 cycles
-Hypofractionated radiotherapy: 45Gy/3Gy/15F
-Prophylactic cranial irradiation: 25Gy/2.5Gy/10F
-Consolidation therapy: serplulimab, 300 mg Q3W, until progression or unacceptable toxicity or up to 1 year
The preliminary analysis aimed to evaluate the efficacy of serplulimab consolidation therapy on the objective response rate (ORR), depth of response (DpR), disease control rate (DCR), survival outcomes, and safety profile in LS-SCLC patients.
Patients Between May 2022 and August 2023, 55 patients were enrolled. The date of data cut-off was April 7, 2024, and the median follow-up duration since initiating serplulimab was 9.8 months. The median cycles of consolidation therapy were 8, and 9 (16.4%) patients had completed the prescribed treatment.
Basic characteristics
Efficacy The ORR and DCR following serplulimab treatment were both 96.4% (95% CI 87.5-99.6). The tumor responses were assessed following serplulimab treatment compared with pre-chemoradiotherapy.
Summary of tumor responses
Overall, 90.9% of patients achieved a DpR exceeding 50%, and patients achieved sustained tumor remission following serplulimab consolidation therapy.
PFS from initiation of consolidation therapy
The median progress-free survival (PFS) was not reached, and 1-year PFS rate was 71.8% (95% CI 60.1-85.8).
Safety Four patients discontinued the study due to TRAEs. Pneumonitis (5.45%) was the most common grade 3-4 TRAEs. No treatment-related death event was reported.
Summary of AEs in consolidation therapy
Conclusion These results highlight the potential survival benefit and manageable safety of consolidation serplulimab therapy in LS-SCLC.
A Real-world Retrospective Analysis Efficacy and Safety of Integrating Consolidative Thoracic Radiotherapy with Immunochemotherapy in ES-SCLC: A Real-world Retrospective Analysis Study Design This single-center retrospective study analyzed the medical records of patients diagnosed with ES-SCLC at Shandong Cancer Hospital between January 1, 2022 and December 31, 2023. Patients were stratified into three cohorts based on the first-line treatment received: chemoradiotherapy (cohort A), immunochemotherapy (cohort B), and immunochemotherapy followed by cTRT (cohort C). Propensity score matching (PSM) was utilized to adjust for baseline differences. Primary outcomes include real-world PFS (rwPFS) and OS, and secondary outcomes include real-world safety profile.
Of the 374 patients analyzed, cohort C showed significant improvements in rwPFS and OS compared to cohort A. The median rwPFS in cohort C (10.9 months) was longer than cohort A (7.6 months) and B (8.0 months). The 12-month rwPFS rate was highest in cohort C (41%), compared to cohort A (19%) and cohort B (34%). Median OS (mOS) was 14.0 months in cohort A, 20.8 months in cohort B, and not reached in cohort C, with the lower limit of the 95% CI for cohort C being 17.80 months. The 18-month OS rate in cohort C (63%) was numerically higher than both cohort A (28%) and B (62%). After propensity score matching, cohort C still showed improvements in rwPFS and OS. The incidence of grade 3 or higher adverse events was comparable across cohorts, with myelosuppression being the most common. However, the incidence of grade 3 or higher pneumonitis was notably higher in cohorts B and C, aligning with previous reports.
Real-world PFS Kaplan-Meier Curvesfor All Patients Included in the Study Across the Three Cohorts
OS Kaplan-Meier Curves for AllPatients Included in the Study Across the Three Cohorts
Incidence and Severity of SpecificInterested Adverse Events Across the Three Cohorts
Conclusion Over half of the patients in the immunotherapy cohorts (B and C) received serplulimab (an anti-PD-1 monoclonal antibody). The combination of cTRT with immunochemotherapy for ES-SCLC showed improved rwPFS and OS, and the overall safety profile remained manageable. These findings highlight the need for further prospective studies to confirm the optimal integration of cTRT in ES-SCLC treatment strategies.
An Open-label, Single-arm, Phase 2 Trial Perioperative serplulimab and chemotherapy in patients with resectable squamous non-small cell lung cancer: an open-label, single-arm, phase 2 trial Study Design This open-label, single-arm, phase 2 trial was conducted to evaluate the efficacy of perioperative serplulimab in patients with resectable squamous NSCLC.
The inclusion criteria were:
-Age: between 18 and 75 years
-Surgically resectable stage II-IIIA squamous NSCLC.
Patients were given intravenous serplulimab (300mg) in combination with (nab—) paclitaxel and carboplatin in 21-day cycles. Neoadjuvant therapy consisted of 2-3 cycles, while adjuvant therapy comprised 1-2 cycles, for a total of 4 cycles.
The primary endpoint of this study was the major pathological response (MPR), defined as 10% or less viable tumor cells in the resected specimen.
The secondary endpoint was pathological complete response (pCR), defined as the absence of viable tumour cells in the resected specimen.
A total of 30 patients were enrolled. Among them, 16 (53.3%) patients had stage IIIA disease. Surgical resection was performed in 29 (97%) patients, with R0 resection achieved in 28 (93.3%) patients.
MPR was observed in 23 (76.7%, 95%CI: 57.3-89.4%) patients, and pathological complete response (pCR) occurred in 15 (50%, 95%CI: 31.7-68.3%) patients.
Of the 30 patients, 2(6.67%) achieved CR, 20 (66.67%) achieved PR, 8(26.67%) achieved SD, and the ORR was 73.33%.
Neutropenia was the most prevalent grade 3-4 adverse events (13 [43%] of 30 patients).
The clearance of circulating tumor DNA following neoadjuvant treatment was found to be a significant predictor of pCR (P=0.007), with a sensitivity of 78.6% and specificity of 76.9%.
Conclusion The exploratory biomarker analysis suggests improved clinical benefit with the addition of serplulimab to chemo regardless of genetic mutation status. Furthermore, comparing to those without mutations, patients with mutations in Notch signalling pathway, KMT2D, PIK3C2G, or EPHA3 may derive more clinical benefit when serplulimab was added.
Neoadjuvant Treatment in HER2-positive, Stage II-III Breast Cancer Phase II study of pyrotinib plus albumin-bound paclitaxel and Trastuzumab (HLX02) as neoadjuvant treatment in HER2-positive, stage II-III breast cancer Methods Patients with previously untreated non-metastatic HER2-positive BC were assigned to receive six neoadjuvant cycles of oral pyrotinib (400 mg) once daily, plus HLX02 (8 mg/kg loading dose, followed by 6 mg/kg) and Nab-P (260 mg/m2) q3w. The primary endpoint was pCR (ypT0/isN0). CTCs was captured and counted at baseline (P0), after neoadjuvant therapy (P1), and after surgery (P2) using Metafer-SE-iFish, and analyzed using Imaris 10.1 following image acquisition with the BZ-X800 microscope.
From December 2020 to August 2023, a total of 214 patients were enrolled and completed neoadjuvant therapy and surgery. The pCR rate was 62.96% (95% CI, 55.65% -69.86%, p=. 001). The most common grade 3-4 adverse events were diarrhea (34.7%) and neutropenia (6.5%). Of 95 patients who underwent CTCs analysis, 63 (66.3%) achieved pCR. CTCs count was significantly reduced at P1 compared to that of at P0 (3.06±3.38 vs.0.84±1.53 FU/5ml, p<.001). Patients with pCR have larger nuclei size at P0 (95% CI, 1.02-1.38; P =. 04), less count (95% CI, 1.49-8.28; P =. 03) and lower membrane intensity (95% CI,0.93-0.98; P =. 03) at P1 to those with non-pCR (Table).
CTCs Univariate and multivariate analysis with pCR for HER2-positive, stage II-III breast cancer
Conclusion In women with HER2-positive, stage II-III BC, the neoadjuvant regimen pyrotinib plus Nab-P and HLX02 effectively promoted pCR of tumor and presented an acceptable tolerability. Personalized monitoring of CTCs during neoadjuvant therapy of early BC may aid in real-time assessment of treatment response and help predict pCR.
0人
- 每日推荐
- 股票频道
- 要闻频道
- 港股频道
 A股上市公司分红生态出现新变化
A股上市公司分红生态出现新变化
 产业优势锻造外贸“爆发力”
产业优势锻造外贸“爆发力”
- 美联储降息50基点 开启降息周期
- 工信部征求意见:电动自行车最高设计车速不应超过25km/h
- 刚刚!人民币大涨,发生了什么?
- 涨停复盘:沪指放量反弹收涨0.69% 两市近4800家个股上涨
- 今日复牌!“中国巨轮”重磅重组
- 中证海外中国内地企业互联互通信息技术指数报843.00点,前十大权重包含比亚迪电子等
- 中证海外中国内地企业互联互通能源指数报1172.39点,前十大权重包含兖矿能源等
- 中证汽车指数报5521.38点,前十大权重包含宇通客车等
- 机构论市:预计美联储仍将继续降息

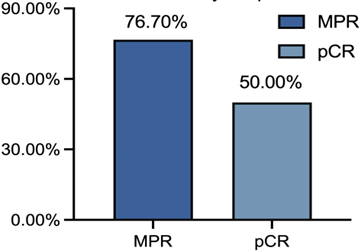
 国家外汇局:跨境资金流动更趋稳定
国家外汇局:跨境资金流动更趋稳定
 探索具有中国特色的数据要素价值化之路
探索具有中国特色的数据要素价值化之路
 23泰达投资MTN004:回售金额5.27亿元,将进行转售
23泰达投资MTN004:回售金额5.27亿元,将进行转售
 快手高级副总裁盖坤:可灵AI正在内测全新的1.5版本基础模型
快手高级副总裁盖坤:可灵AI正在内测全新的1.5版本基础模型



